The Gastrolab Image Gallery
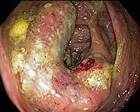
A Caecal Adenocarcinoma and Pseudomelanosis Coli
![]()
This free script provided by
JavaScript
Kit
Here an article dealing with the use of senna and risk of cancer:
J Toxicol. 2009; 2009: 287247.
Is Senna Laxative Use Associated to Cathartic Colon, Genotoxicity, or Carcinogenicity?
M. A. Morales,1* D. Hernández,2 S. Bustamante,1 I. Bachiller,3 and A. Rojas4 1Departamento de Farmacologia, Instituto de Ciencias Biomédicas, Facultad de Medicina, Universidad de Chile, 8380453 begin_of_the_skype_highlighting 8380453 end_of_the_skype_highlighting Independencia, Santiago, Chile 2Departamento de Ciencias Biológicas, Facultad Ciencias de la Salud, Universidad Andrés Bello, República 217, Santiago, Chile 3Sociedad Asturiana de Fitoterapia, 33005 Oviedo, Asturias, Spain 4Facultad de Ciencias de la Salud, Escuela de Medicina, Universidad Católica del Maule, Casilla 617, Talca, Chile
Recommended by Michael Cunningham
Received June 8, 2009; Accepted August 11, 2009.
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract:
Due to their natural origin, apparent low oral toxicity, effectiveness, and accessibility without a medical prescription, the anthranoid laxatives are a popular remedy for constipation and are frequently used abusively. Therefore, it is important to characterize its harmful and/or toxic effects. The sennosides, main active metabolites of senna, exhibit a very low toxicity in rats, and its genotoxic activity in bacterial strains as well as mammal cells was classified as weak in those cases where it was shown to be significant. The toxicological and mutagenic status of the crude extract of senna, however, is not as well characterized, and it is necessary to do so since it is frequently, and at the same time incorrectly, believed that the chronic use of anthranoid laxatives is a risk factor for the development of colorectal cancer. The objective of this article was to review the information that arises in various scientific medical databases using key words such as senna, sen, Senna alexandrina, Cassia angustifolia, sennosides, laxative toxicity, mainly ISI and non-ISI articles of journals with an editorial committee. Web pages of products or companies that publicize or commercialize this type of laxative were not included. This analysis establishes that (1) there is no convincing evidence that the chronic use of senna has, as a consequence, a structural and/or functional alteration of the enteric nerves or the smooth intestinal muscle, (2) there is no relation between long-term administration of a senna extract and the appearance of gastrointestinal tumors or any other type in rats, (3) senna is not carcinogenic in rats even after a two-year daily dose of up to 300 mg/kg/day, and (4) the current evidence does not show that there is a genotoxic risk for patients who take laxatives containing senna extracts or sennosides.
You might also be interested in:
 Colon Cancer (Adenocarcinoma), Ascending Colon
Colon Cancer (Adenocarcinoma), Ascending Colon |
 A Colon Cancer (Adenocarcinoma)with Chicken Skin Mucosa
A Colon Cancer (Adenocarcinoma)with Chicken Skin Mucosa |
 A Colon Cancer (Adenocarcinoma)
A Colon Cancer (Adenocarcinoma) |
 A Typical Colon Cancer (Adenocarcinoma)
A Typical Colon Cancer (Adenocarcinoma) |
 Polypoid Cancer in the Transverse Colon
Polypoid Cancer in the Transverse Colon |
 VIDEO: Caecal Cancer (Adenocarcinoma)
VIDEO: Caecal Cancer (Adenocarcinoma) |
 VIDEO: Caecal Cancer (Adenocarcinoma)
VIDEO: Caecal Cancer (Adenocarcinoma) |
 VIDEO: Caecal Cancer (Adenocarcinoma) and Pseudomelanosis
VIDEO: Caecal Cancer (Adenocarcinoma) and Pseudomelanosis |
 VIDEO: Colon Cancer (Adenocarcinoma) in the Ascending Colon
VIDEO: Colon Cancer (Adenocarcinoma) in the Ascending Colon |
 VIDEO: Colon Cancer (Adenocarcinoma) in the Ascending Colon
VIDEO: Colon Cancer (Adenocarcinoma) in the Ascending Colon |
|
|

